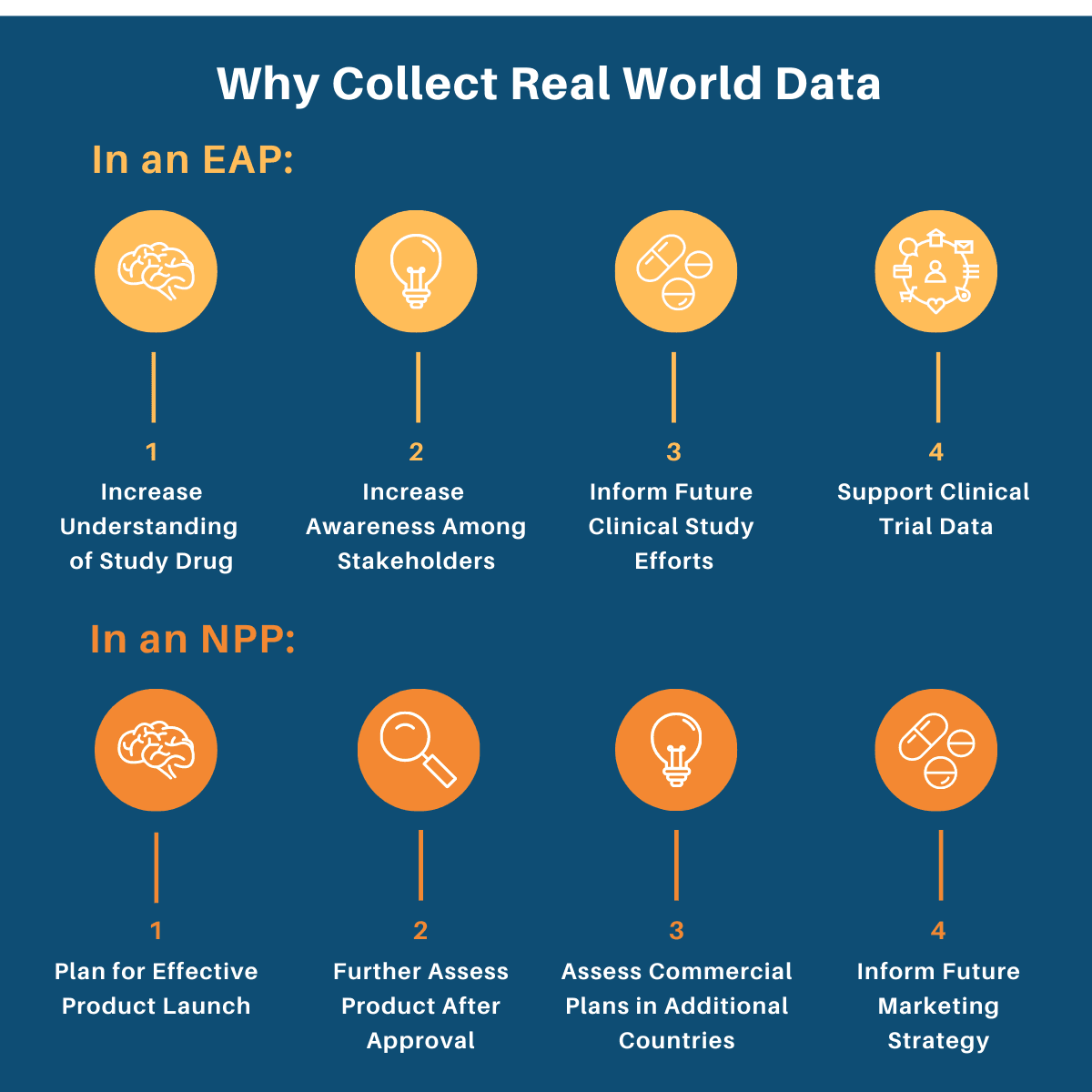
why collect Real world data in an expanded access or named patient program?
Expanded Access (EAPs) and Named Patient Programs (NPPs) provide sponsors the opportunity to collect meaningful Real World Data (RWD), i.e. data on health care that is derived from multiple sources outside of the typical clinical research settings.
More and more, regulatory bodies around the world, like the US Food and Drug Administration (FDA), European Medicines Agency (EMA) and the Australian Therapeutic Goods Administration (TGA), etc. are encouraging companies to collect RWD, if and when appropriate. These regulators recognize the tremendous value in collecting data in the real world setting and using it to better inform stakeholders and promote safe and effective use of products, quicker, for patients in need.
At WEP Clinical, we work closely with our sponsor companies to determine if and how RWD collection can be incorporated into their EAP or NPP. This involves understanding the various motivations behind collecting RWD in these two types of programs, as data can be analyzed and used for a variety of different purposes.
Below we have outlined some of the reasons sponsors might choose to collect RWD in an EAP or an NPP.